How Do You Know if Somethings Is Soluble
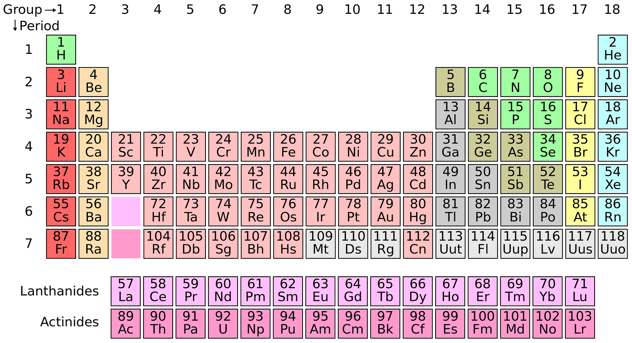
One of the offset scientific discipline experiments I remember was adding salt to a cup of water and waiting eagerly for it to dissolve. Though I was excited to watch the table salt seem to "disappear" I definitely didn't empathize the intricacies of solubility. Luckily, solubility follows a list of rules that helps us determine how soluble a substance is, like how probable that salt is to dissolve into that water (sneak peek- it's very likely). Nosotros're going to go over what solubility is, how information technology works, and the consummate list of solubility rules to help you determine the solubility of substances. Solubility is a substance'south ability to be dissolved. The substance that is dissolved is called a solute, and the substance information technology is dissolving in is called a solvent. The resulting substance is called a solution. Generally, the solute is a solid and the solvent is a liquid, such every bit our common salt in water example above. Withal, solutes can exist in any land: gas, liquid, or solid. For example, a carbonated beverage is a solution where the solute is a gas and the solvent is a liquid. A solute is considered insoluble when they are unable to dissolve at a ratio greater than 10000:1. While many compounds are partially or mostly insoluble, in that location is no substance that is completely insoluble in water, significant that it tin't deliquesce at all. You lot will run across in the solubility rules that many compounds that are labeled as insoluble have exceptions, such as carbonates. This is partly why it's important to follow the solubility rules closely. When you are working on chemical equations or edifice a hypothesis, solubility rules are helpful in predicting the stop states of the substances involved. Y'all will be able to accurately predict what combinations will lead to what results. The solubility rules are just for ionic solids' ability to dissolve in water. While we can calculate the solubility by measuring each substance and following an equation, the solubility rules let the states to determine the solubility of a substance before you try to create information technology. It is very important that the rules on this list are followed in order, because if a rule seems to contradict another rule, the dominion that comes start is the 1 that you follow. Substances on this list are given past their elemental names. Referencing the periodic table beneath will help you piece of work through the elemental names and groups. Salts containing Group I elements (Li+, Na+, K+, Cs+, Rb+) are soluble . At that place are few exceptions to this rule. Salts containing the ammonium ion (NH4+) are as well soluble. Salts containing nitrate ion (NO3-) are generally soluble. Salts containing Cl -, Br -, or I - are more often than not soluble. Important exceptions to this dominion are halide salts of Ag+, Pb2+, and (Hg2)2+. Thus, AgCl, PbBr2, and Hg2Cl2 are insoluble. Near silver salts are insoluble. AgNO3 and Ag(C2H3O2) are common soluble salts of silver; near all others are insoluble. Most sulfate salts are soluble. Important exceptions to this rule include CaSO4, BaSO4, PbSO4, Ag2SO4 and SrSO4 . About hydroxide salts are only slightly soluble. Hydroxide salts of Grouping I elements are soluble. Hydroxide salts of Grouping Ii elements (Ca, Sr, and Ba) are slightly soluble. Hydroxide salts of transition metals and Al3+ are insoluble. Thus, Iron(OH)three, Al(OH)3, Co(OH)2 are not soluble. Most sulfides of transition metals are highly insoluble, including CdS, FeS, ZnS, and Ag2S. Arsenic, antimony, bismuth, and lead sulfides are also insoluble. Carbonates are ofttimes insoluble. Grouping II carbonates (CaCO3, SrCO3, and BaCO3) are insoluble, as are FeCO3 and PbCO3. Chromates are frequently insoluble. Examples include PbCrO4 and BaCrO4. Phosphates such as Ca3(PO4)2 and Ag3PO4 are often insoluble. Fluorides such as BaF2, MgF2, and PbF2 are frequently insoluble. 1. Select the compounds that are always soluble in water a. BaSO4 b. HG2 I2 c. Na OH d. Na2 SO3 e. Ag ClO3 f. Cr Cl3 g. Atomic number 26 PO4 ii. Label each of the following every bit soluble or insoluble a. Li OH b. Hg SO4 c. Pb Br2 d. Rb2 South eastward. Ni I2 f. H3 AsO4 g. Ni Cro4 iii. Which (if any) argent is soluble: Silver chloride AgCl, silver phosphate, Ag3 PO4, or silver fluoride, AgF? 1. Select the compounds that are always soluble in water (bolded are right) a. BaSO4 (run across dominion 5) b. HG2I2 (run across rule 3) c. Na OH (meet rule one) d. Na2 SO3 (see rule 1) due east. Ag ClO3 (see dominion iii) f. Cr Cl3 (come across rule 3) g. Fe PO4 (run across dominion six) Note: Letter of the alphabet e is an example of using the lodge of the rules to determine solubility. Rule 4 says that silvers (Ag) are frequently insoluble, but rule iii says that chlorates (Cl) are soluble. Since Ag ClO3 is a silver chlorate, and rule iii comes before rule 4, information technology supersedes it. This compound is soluble. two. Label each of the following every bit soluble or insoluble a. Li OH soluble- rule i b. Iron (OH)2 insoluble- rule vii c. Lead Br2 insoluble – rule 2 d. Rb2 SO3 soluble- rule 1 east. Ni I2 soluble – rule 3 f. H3 AsO4 insoluble- rule 10 one thousand. Ni CRo4 insoluble- rule viii 3. Which (if whatever) silver is soluble: Silver chloride AgCl, silvery phosphate, Ag3 PO4, or silverish fluoride, AgF? None of the in a higher place silver is soluble. In rule #4, it states that silver salts (Ag) are Every bit we see from our solubility rules, some substances are very soluble, while some are insoluble or have low solubility. Permit's take a look at how solubility works to improve sympathize the solubility rules. Whether or not a substance is soluble, and to what caste, depends on a variety of factors. Solutes typically will dissolve best in solvents that accept the well-nigh molecular similarities. Polarity is a major factor in a substance's solubility. Molecules where one stop is negatively charged and the other is positively charged are considered "polar," meaning that they take electric poles. If a molecule does not take this ionic makeup, information technology is considered nonpolar. By and large, solutes are soluble in solvents that are well-nigh similar to them molecularly. Polar solutes volition dissolve better in polar solvents, and non-polar solutes will dissolve better in non-polar solvents. For example, sugar is a polar solute, and absorbs very well in water. Notwithstanding, sugar would have a low solubility in a nonpolar liquid similar vegetable oil. In general, solutes volition also be more soluble if the molecules in the solute are smaller than the ones in the solvent. Other factors that affect solubility are pressure and temperature. In some solvents, when heated the molecules vibrate faster and are able to pause autonomously the solute. Pressure is mainly a factor when a gas substance is involved, and has little to no effect on liquid substances. The rate of solution refers to how speedily a substance dissolves, and is separate from solubility. Solubility depends entirely on the physical and chemical properties of the solute and solvent, and isn't afflicted by the rate of solution. Rate should not be factored into the solubility of a substance.This can often exist confusing when kickoff learning about solubility, since in a visual instance, watching something dissolve apace can feel similar an affidavit of its ability to deliquesce. However, the process of solubility is unique, and the rate at which it dissolves is not factored into the equation. When a solute is mixed with a solvent, there are three possible outcomes: If the solution has less solute than the maximum amount it is able to dissolve (the solubility), it is a dilute solution. If the amount of solute is exactly the same equally the solubility it is saturated. If at that place is more solute than is able to be dissolved, the excess separates from the solution and forms a precipitate. A solution is considered saturated when adding additional solute does not increment the concentration of the solution. Additionally, a solution is miscible when it can be mixed together at any ratio- this mainly applies to liquids, like ethanol, C2H5OH, and water, H2O. Knowing and post-obit the solubility rules is the all-time way to predict the effect of any given solution. If nosotros know that a substance is insoluble, it is likely that it would accept excess solute, thus forming a precipitate. All the same, compounds that nosotros know to be highly soluble, like salt, are probable to course solutions at various ratios; in this example, nosotros will exist able to determine how much solute and solvent is needed to form each solution, and if it's possible to form i at all. Thinking about the salt in water experiment now, it's articulate that the salt- too known as NaCl or sodium chloride, would exist highly soluble according to our solubility rules. Sodium chloride contains Na, which is near ever soluble according to rule 1, and Cl, which is usually soluble co-ordinate to rule 3. Though I can tell this just by glancing at the rules, null takes away from the magic of watching chemic compounds interruption downwards and dissolve correct before your eyes. Call back to go on your periodic tables handy, and pay close attention to the solubility rules in your next experiment. Preparing for the AP Chemistry test? Study with our manufactures on every AP Chemical science practice examination bachelor and the ultimate AP Chem written report guide. Taking IB instead? Start with our study notes for IB Chemistry. Looking for more than chemistry help? We walk you through the solubility abiding (K sp ) and how to solve for it, explain how to remainder chemical equations, and go over examples of physical vs chemical change here. If you need more non-chemistry science guides, be sure to check out these guides about finding the density of h2o, defining commensalism, and how to summate acceleration.
What Is Solubility?
Solubility Rules
Sample Questions
Answers
insoluble, with silver nitrate, AgNO3, equally one exception.
How Solubility Works
Factors That Touch on Solubility
Predicting Outcomes
What's Next?
Carrie holds a Bachelors in Writing, Literature, and Publishing from Emerson College, and is currently pursuing an MFA. She worked in book publishing for several years, and believes that books can open upwards new worlds. She loves reading, the outdoors, and learning about new things.
Source: https://blog.prepscholar.com/solubility-rules-chart-chemistry
0 Response to "How Do You Know if Somethings Is Soluble"
Post a Comment